The Results of Asandeutertinib Tablets (TY-9591) will be Presented at the WCLC 2024
Time:2024/09/06
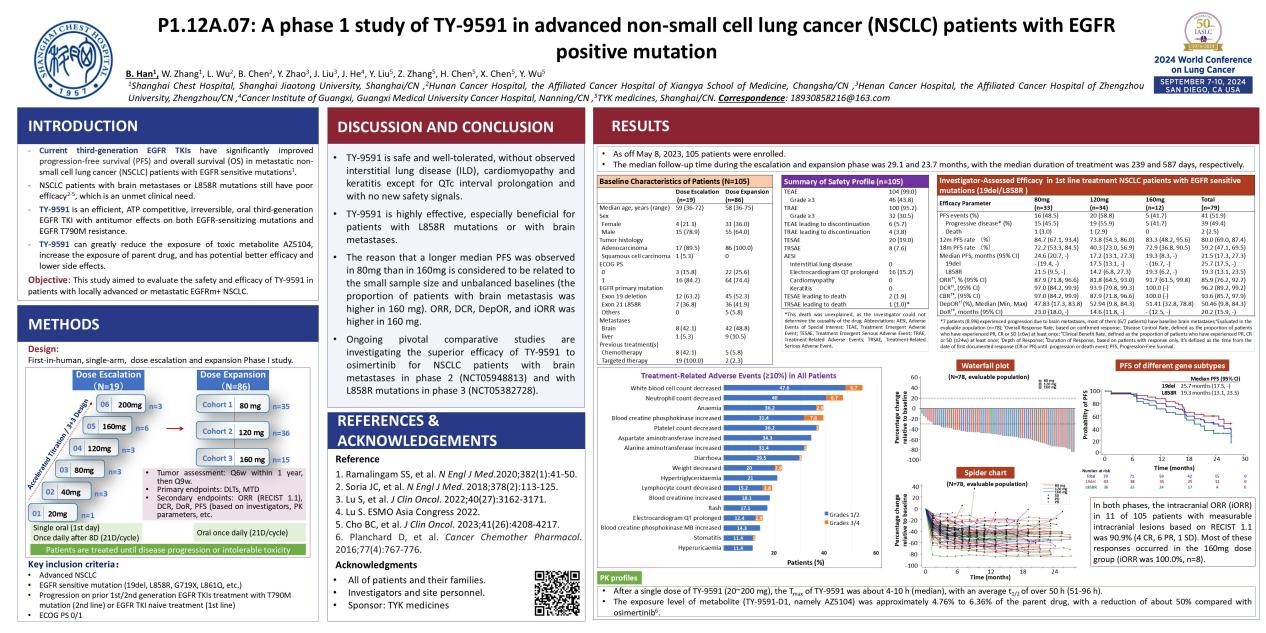
IASLC 2024 World Conference on Lung Cancer (WCLC) will be held from September 7-10 in San Diego, USA. As the annual event in the field of lung cancer, numerous significant research results will be presented. The phase Ⅰ study results of the innovative drug asandeutertinib tablets (TY-9591) developed by TYK medicines to treat advanced EGFR mutation-positive NSCLC will be presented at this conference.
Abstract number: P1.12A.07
Type: Poster
PI: Professor Han Baohui, Shanghai Chest Hospital
Background
EGFR-TKIs have shown significant efficacy in patients with EGFR-sensitive mutation NSCLC, improving patients’ prognosis and extending overall survival. However, they have limited efficacy in NSCLC patients with brain metastases, which severely threaten life and affect the quality of life, meanwhile, their efficacy in EGFR/L858R mutation patients is far lower compared to those with 19del mutation patients. Therefore, there remain unmet clinical needs for NSCLC patients with brain metastases and the EGFR/L858R mutation population.
Study Design
This study is a single-arm, open-label, multi-center phase I clinical trial, including a dose escalation stage (phase Ia) and a dose expansion stage (phase Ib). It mainly includes advanced NSCLC patients with EGFR-sensitive mutations who have progressed after first-line standard treatment (second-line) or have not received EGFR TKIs treatment (first-line). In phase Ia, an accelerated titration method (20 mg) combined with the “3+3” method was used to explore the escalation of six dose groups (20/40/80/120/160/200 mg). TY-9591 was given as a single oral dose on the first day, followed by once daily on the 8th day (21 days/cycle). In phase Ib, three dose groups (80/120/160 mg) were expanded, with once-daily oral administration (21 days/cycle). Patients were treated until disease progression or intolerable toxicity occurred.
Safety
All six dose groups completed escalation (19 patients with EGFR/T790M+ NSCLC ), and no dose-limiting toxicities (DLTs) were observed.
Among the 105 enrolled patients, the incidence of adverse reactions during treatment was 95.2%, with the most common being hematologic toxicities such as decreased white blood cell count, decreased neutrophil count, and anemia. No adverse events of special interest (AESI) such as interstitial lung disease, cardiomyopathy, or keratitis were observed. The incidence of permanent discontinuation was 3.8%, and the incidence of serious adverse reactions (SAR) was 7.6%.
Efficacy
The median PFS of TY-9591 as a first-line treatment for EGFR-sensitive mutation (19del or L858R) NSCLC patients was 21.5 months, with 25.7 months in the 19del subgroup and 19.3 months in the L858R subgroup.The confirmed ORR was 85.9%, DCR was 96.2%, tumor response depth (DepOR) was 50.46%, and the median DoR was 20.2 months.
In phase Ia and Ib, the proportions of brain metastasis patients were 42.1% and 48.8%, respectively. Eleven patients had measurable intracranial lesions. The intracranial ORR (iORR) based on RECIST 1.1 was 90.9% (4 CR, 6 PR, 1 SD), with most responses occurring in the 160 mg dose group (iORR was 100%, n=8).
Pharmacokinetics
The exposure (AUC) of the toxic metabolite TY-9591-D1 was about 4.76-6.36% of the parent drug under steady state.
Conclusion
The results of this study indicate that TY-9591 is safe and tolerable, with no special safety signals observed, and showing significant efficacy, particularly in NSCLC patients with brain metastases and EGFR/L858R mutation. Currently, pivotal phase II and III clinical studies (NCT05948813, NCT05382728) of TY-9591 are ongoing.
