TYK Medicine TYK-00540—The first CDK2/4/6 inhibitor in China approved by FDA to conduct clinical trial
Time:2023/06/21
On June 21, 2023, TYK Medicines announced that its independently developed and effective oral next-generation Cyclin-dependent kinase2/4/6 (CDK2/4/6) inhibitor TYK-00540 received the official approval letter (Study May Proceed Letter) from the U.S. Food and Drug Administration (FDA) for conducting clinical trials.
Currently, there is a significant unmet clinical demand as no CDK2/4/6 inhibitors have been authorized for marketing. This is another important milestone for TYK to focus on the research and development of innovative small-molecule targeted tumor drugs and differentiated product layout in the field of solid tumors such as breast cancer.
About TYK-00540
TYK-00540 is a novel, highly effective and small-molecule inhibitor of CDK2/4/6, independently developed by TYK Medicines, Inc, aiming for various advanced tumors, such as breast cancer and ovarian cancer. TYK-00540 has demonstrated marked sensitivity of many types of cancer towards CDK2/4/6 inhibition via preclinical animal models, as well as high tolerance and safety, laying a solid foundation for future clinical practice.
About CDK2/4/6
Cyclin-dependent kinases (CDKs) are serine/threonine kinases whose catalytic activities are regulated by interactions with cyclins and CDK inhibitors (CKIs). CDKs are key regulatory enzymes involved in cell proliferation via regulating cell-cycle checkpoints and transcriptional processes in response to extracellular and intracellular signals [1]. CDKs respond to the extracellular and intracellular signals to regulate cell division, acting as the catalytic subunits. In human cells, there are 20 CDKs and 29 cyclins. CDK1, CDK2, CDK3, CDK4, CDK6 and CDK7 directly regulate cell-cycle transitions and cell division [1]. Although CDK4/6 inhibitors have significantly altered treatment scheme of HR+/HER2- breast cancer, there are still considerable challenges, mainly manifested in primary and acquired CDK4/6 inhibitors resistance [2].
The published study showed that when CDK4/6 activity was inhibited, with the expansion of Cyclin E and activation of MYC, CDK2 was upregulated by MYC, and CDK2-CyclinE could be used as a compensatory pathway to phosphorylate Rb and release E2F to promote the proliferation of tumor cells, which is the main mechanism of acquired drug resistance of CDK4/6 inhibitors [3]. Overexpression of Cyclin E makes tumor cells resist the inhibitory effect of CDK4/6 and no longer stagnate in the G1 phase [4]. Patients with high Cyclin E expression are insensitive to CDK4/6 inhibitors, and their progression-free survival is significantly shorter than that of Cyclin E amplification induced resistance. This mechanism has been confirmed in CDK4/6 resistant cell lines [5]. To achieve long-term efficacy, both CDK4 and CDK2 need to be inhibited, which is a new type of treatment that inhibits cancer cells. Simultaneous targeting of CDK2/4/6 is expected to reduce the occurrence of CDK4/6 inhibitory resistance.
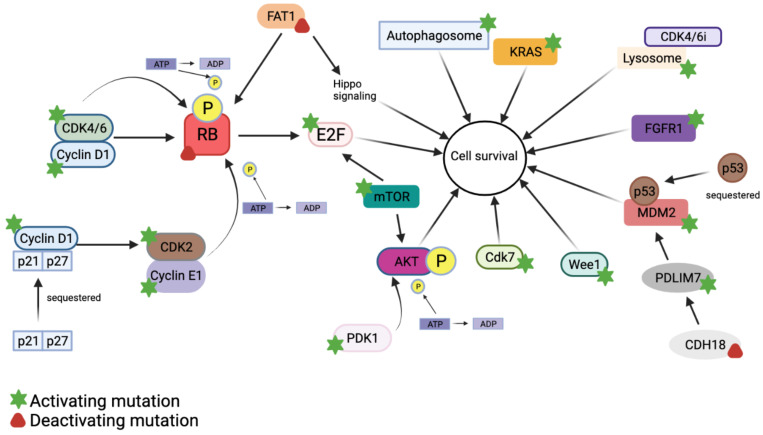
Figure 1: Mechanism of CDK4/6 inhibitor resistance
References
[1]. Ding L, Cao J, Lin W, et al. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int J Mol Sci. 2020;21(6):1960. Published 2020 Mar 13. doi:10.3390/ijms21061960.
[2]. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35(32):3638-3646. doi:10.1200/JCO.2017.75.6155.
[3]. Freeman-Cook K, Hoffman RL, Miller N, et al. Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell. 2021;39(10):1404-1421.e11. doi:10.1016/j.ccell.2021.08.009.
[4]. Freeman-Cook KD, Hoffman RL, Behenna DC, et al. Discovery of PF-06873600, a CDK2/4/6 Inhibitor for the Treatment of Cancer. J Med Chem. 2021;64(13):9056-9077. doi:10.1021/acs.jmedchem.1c00159.
[5]. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417-430. doi:10.1038/nrclinonc.2016.26.
