2025 02
25

2024 09
06

The Results of Asandeutertinib Tablets (TY-9591) will be Presented at the WCLC 2024
Discover More
2024 01
04

TYK Medicines Announced First Patient Dosed in Phase 1 clinical trial of its CDK2/4/6 inhibitor TYK-00540
Discover More
2023 09
22

TY-2136b granted Orphan Drug Designation in the US for Non-Small Cell Lung Cancer
Discover More
2023 09
08
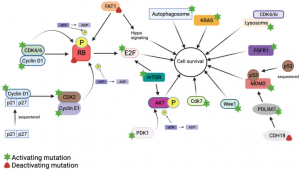
TYK Medicine TYK-00540-The first CDK2/4/6 inhibitor in China approved by FDA to conduct clinical trial
Discover More
2023 08
18


